A Review of the Biomechanics of the Diabetic Foot
Introduction
Diabetic pes ulcers remain one of the most serious complications of diabetes mellitus (Burns and Jan, 2012). Information technology is estimated that 15% of diabetics will develop a foot ulcer during their lifetime, and two–3% of the population may develop a foot ulcer annually (Burns and Jan, 2012). In 2000–2001, the cost of treating a diabetic foot ulcer averaged $xiii,179 per episode, which increased with severity level (Stockl et al., 2004). In 2007, the full cost of treatment was $58 billion in the United States (American Diabetes Association, 2008). Diabetic peripheral neuropathy causes not only loss of protective sensation but also changes in the soft tissues of the foot likewise equally dryness of the skin that can lead to excessive formation of callus (Burns and Jan, 2012; January et al., 2013a,b). These changes affect ambulatory function that may lead to high plantar pressures in diabetics (Lung and Jan, 2012; Jan et al., 2013a). The repetitive high pressure insults to the plantar surface of the diabetic foot have been shown to exist associated with the development of human foot ulcers (Veves et al., 1992; Bus, 2012; Patry et al., 2013).
To improve understand the influence of aberrant plantar pressure distributions on the development of diabetic pes ulcers, peak plantar pressure level (PPP) has been widely used to assess trauma to the soft tissues of the diabetic foot (Veves et al., 1992; Pitei et al., 1999b; Caselli et al., 2002). Still, the threshold of the PPP for causing diabetic foot ulcers remains largely unknown (Armstrong et al., 1998a; Mak et al., 2010). Furthermore, only a moderate correlation between the location of diabetic foot ulcers and the PPP has been reported (Veves et al., 1992). Lavery et al. (2003) suggested that the PPP alone is not adequate to predict the evolution of skin breakdown; they suggested that other variables and methods should be investigated to predict the risk of diabetic foot ulcers. Because of the circuitous geometry and not-linear material properties of the human foot, the forces, pressures, and stresses acting on the plantar soft tissues showroom a complex behavior (Gefen et al., 2000; Chen et al., 2010; Jan et al., 2013a).
Mueller et al. (2005) introduced another index, peak pressure slope (PPG), for characterizing the largest change in plantar force per unit area between adjacent pressure sensors pixels of a pressure mapping system. Thus, the PPG is a metric that captures the largest pressure level gradient observed in a given region of the plantar surface. The PPG has shown promise in predicting the development of diabetic human foot ulcers in many previous studies (Mueller et al., 2005, 2008; Zou et al., 2007; Lott et al., 2008), and diverse ethnic people (Lung et al., 2013; Fawzy et al., 2014). Supriadi et al. (2014) further defined a cutoff value of PPG for the risk threshold of pressure ulcers.
According to the principle of PPG, a low average PPP coupled with high PPG is more dissentious than a high PPG on its own. Lott et al. (2008) demonstrated a pregnant human relationship among PPP, PPG, and maximal shear stress in the diabetic foot. The high PPG may contribute to skin breakdown considering high PPG may crusade large shear stresses within the plantar soft tissues (Mueller et al., 2005). Jan et al. (2013a) further demonstrated that changes in viscoelastic properties of plantar soft tissues contribute to abnormal PPP and PPG patterns in diabetics with peripheral neuropathy. Due to the circuitous dynamics experienced by the ankle and human foot during gait and the structural and functional changes associated with diabetes, the current definition of PPG may non be adequate to identify diabetics at risk of diabetic foot ulcers (Jan et al., 2013a).
Co-ordinate to the definition of PPG proposed past Mueller et al. (2005), the PPG is calculated based on PPP distributions during the overall contact time without consideration of time-varying features of PPP locations during the gait cycle. Still, the directions of consecutive maximal pressure gradients may vary during gait. Therefore, PPG by itself does not account for variations in force per unit area gradient direction during the stance stage of the gait bicycle, and varying pressure slope management may cause a more complicated deformation on the underlying plantar soft tissues. To business relationship for the mean directional variations of the pressure level gradient during the opinion phase, a new metric pressure slope bending (PGA) was defined in our previous written report (Lung et al., 2013). The PGA quantifies the time-varying directions of instantaneous PPG.
Theoretically, the PGA increases with the dispersion of pressure (Figure 1B) and decreases with the concentration of pressure (Figure 1C). Equally shown previously, we demonstrated that the PGA provides boosted information to quantify the pressure slope patterns (Lung et al., 2013). The PGA was found to be significantly smaller for diabetics compared with controls under the showtime toe, thus suggesting a greater concentration of pressure gradient in persons with diabetes. The purpose of the current study was to further quantify the differences between diabetics and healthy controls during walking in different plantar regions at high risk of ulceration. We hypothesized that values of PPP and PPG would be greater and the PGA would be lower in diabetics compared with non-diabetics. Our long-term goal is to further amend our understanding of the part of plantar pressures on the pathogenesis of diabetic pes ulcers.
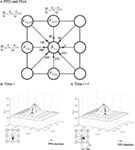
Figure i. Illustrations of the peak pressure gradient (PPG) and pressure gradient angle (PGA). (A) Calculation of the PPG. (B,C) Quantify the time-varying directional variations of pressure gradients (i.e., PGA). The PGA is 45° from (B) time i to (C) time i + i. Although the PPP and PPG values are the same, the directions of PGA are different. The arrow represents the management of peak force per unit area slope.
Materials and Methods
Participants
Twenty-seven volunteers were recruited, including 19 type two diabetics (x males) and eight non-diabetic good for you controls (4 males). Subjects with gross human foot deformities (except minor toe clawing) and prior human foot amputations/major surgeries were excluded for a more homogeneous population. The demographic data of the control group were age 23.i ± iii.2 years, weight 66.8 ± 21.3 kg, acme 1.66 ± 0.12 m, trunk mass alphabetize (BMI) 24.0 ± 6.9 kg/thousand2, eye charge per unit 69.1 ± 7.eight beats/min, systolic blood pressure 108.3 ± 12.7 mmHg, and diastolic blood pressure 68.6 ± viii.seven mmHg. The demographic information of the diabetic group were age 42.2 ± 12.6 years, weight 94.0 ± 21.7 kg, height i.74 ± 0.xv m, BMI 31.iv ± vii.6 kg/g2, heart rate 76.1 ± 13.5 beats/min, systolic blood force per unit area 129.three ± 21.7 mmHg, and diastolic blood pressure 79.2 ± 14.7 mmHg. The fasting blood glucose level and duration of diabetes were 137.half dozen ± x.7 mg/dL and 9.2 ± ii.three years, respectively. All diabetics had plantar pes ulceration in the past and had healed more than 3 months at the time of the experiment; they also had peripheral neuropathy confirmed by the disability to sense a 5.07 Semmes–Weinstein monofilament in at least four locations of the plantar foot (Apelqvist et al., 2000). This report was approved by an institutional review board for human subject research. The research protocol was explained to the volunteers who signed an informed consent form.
Plantar Force per unit area Measurements
The F-scan system (Tekscan, S Boston, MA, U.s.) was used to collect plantar pressure level data of the right pes during walking at a cocky-selected pace (ranged from 2 to 4 km/hr) in standardized shoes (Mueller and Strube, 1996; Pitei et al., 1999b). Each F-scan in-shoe sensor contains 960 sensing pixels (sensels). The size of each pixel is 5.08 mm × 5.08 mm. The sensor was placed between the subject's sock and the insole of the shoe. All subjects wore convalescent shoes with a 1′ heel (Altrex, Teaneck, NJ, USA). The correct shoe was worn with its standard insert and a thin cotton sock. Subjects wore the sensor inside the right shoe for 3–5 min of walking before calibration (Mueller and Strube, 1996; Pitei et al., 1999b). The sensor was then calibrated according to the manufacturer's guidelines and was consequent with utilise reported in the literature (Mueller and Strube, 1996; Pitei et al., 1999b). Information were collected at 200 Hz during 2 walking trials in the same direction on a 15-m walkway immediately after calibration (Mueller and Strube, 1996; Pitei et al., 1999b).
Data Assay and Statistics
Data from the three middle steps were candy to calculate the boilerplate PPP, PPG, and PGA across the three steps. These average values were determined in iv plantar regions at high risk of diabetic foot ulcers (Armstrong et al., 1998a; Lung and Jan, 2012). The four regions were the showtime toe (T1), the first metatarsal caput (M1), the 2nd metatarsal head (M2), and the heel (HL) (Figure 2A) (Armstrong et al., 1998a; Lung and Jan, 2012).
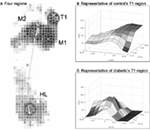
Figure two. Examples of PPP angle at the first toe in a representative control and a representative diabetic. (A) Iv plantar regions are defined. (B) Control: PPP = 262 kPa, PPG = 29 kPa/mm, and PGA = 135°. (C) Diabetic: PPP = 228 kPa, PPG = 58 kPa/mm, and PGA = 0°. For these two sample cases in (B,C), the PPP between the command and diabetic are similar, merely the PPG and PGA are quite different.
The PPP was defined as (Mueller et al., 2005; Zou et al., 2007):
where p is the pressure distribution inside each of the four plantar region surfaces.
Later the maximal value of the p sensor was identified, eight sensors around the p sensor were called. A blended area by these nine sensors was a defined area for the further analyses of all plantar pressure-related variables. Co-ordinate to the convention of Mueller et al., the PPG was determined in a divers expanse [a 3 × 3 box of sensing pixels on the F-browse sensor (232.3 mm2)] around a central node (Effigy 1A). Node positions were generated using a bicubic polynomial spline role (Mueller et al., 2005). The PPG was calculated by determining the greatest divergence in pressure from one node (half sensel apart) to the next according to row, column, and diagonal directions. Thus, each node has eight slope vectors but has one summit vector at the same time, max (Figure 1A). The PPG is the magnitude of the largest acme slope vector for a given gait wheel. The PPG can be calculated equally (Mueller et al., 2005; Zou et al., 2007):
where (∂p/∂r)|(xp, yp ) (space rate of change of pressure level on the plantar surface) is the directional derivative of pressure level p at the node for a given plantar region (xp, yp ) on the plantar surface in any of the eight directions given by the vector .
The PGA can be adamant by because the directional variations of the peak slope vector between two sequent frames of the time-varying, instantaneous PPG, i.e., max and max (Effigy 1A). The angle α can be computed from the dot product of the magnitudes of these 2 vectors. The PGA is the average change in α during a stance phase of gait bicycle (Lung et al., 2013):
where α is the angle of the pressure level gradient vector at time i, and N is the time when the instantaneous PPP is more than half of the overall PPP. As shown in our previous study (Lung et al., 2013), the results of PGA were stable when the PGA was calculated past the instantaneous PPP of more than than fifty% sensors. The selection of pressures with more than half PPP is to exclude unstable PGA associated with pocket-size plantar pressures.
Nosotros analyzed the intra-observer variability by using intraclass correlation coefficients (ICC). The distribution patterns of all variables were analyzed using the one-sample Kolmogorov–Smirnov exam. The differences in the PPP, PPG, and PGA between diabetics and controls were examined using the Student'southward t-test (Klaesner et al., 2002). The values were presented as the mean ± SD. Correlations between the PPP, PPG, and PGA were adamant using a Pearson product-moment correlation analysis (Mueller et al., 2005). The level of the significance was set at 0.05 (Rothman, 1990; Perneger, 1998).
Results
Correlation coefficients among all measurements of intra-observer were high enough (ICC = 0.79). All results of this written report were commonly distributed. Examples of PPP and PPG at the start toe in a diabetic and a healthy control are provided to illustrate the concept of the PGA in Figure 2. Despite that the PPP values of the control and the diabetic subjects were similar (~250 kPa), PPP distributions for controls (Figure 2A) were spatially flatter than diabetic PPP distributions (Figure 2B). These distributions lead to the PPG as 29 kPa/mm in control and 58 kPa/mm in diabetics, respectively. In this case, the PGA was larger at 45° in a healthy control than 0° in a diabetic.
A detailed comparing of differences betwixt the diabetic and control groups and the 4 plantar areas are provided in Table 1. The PPP value at the first toe was significantly smaller in the control grouping (297.0 ± 107.8 kPa) compared with the diabetic group (489.4 ± 211.iv kPa, P < 0.05, Figure 3A). The PPP value at the commencement metatarsal caput was significantly smaller in the control group (319.9 ± 107.8 kPa) compared with the diabetic group (509.6 ± 245.8 kPa, P < 0.05, Figure 3A). The PPG value at the offset toe was significantly smaller in the command grouping (49.0 ± 16.8 kPa/mm) compared with the diabetic grouping (104.9 ± 45.3 kPa/mm, P < 0.05, Figure 3B). The PPG value at the first metatarsal caput was significantly smaller in the command group (47.vii ± 24.4 kPa/mm) compared with the diabetic group (88.3 ± 51.two kPa/mm, P < 0.05, Figure 3B). The PGA at the first toe was significantly greater in the control group (44.0° ± 32.2°) compared with the diabetic group (20.four° ± 22.0°, P < 0.05, Effigy 3C). No significant differences were found for the other 2 areas, second metatarsal head and heel.
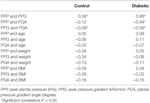
Table i. Correlations among variables in the command and diabetic groups.
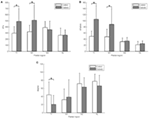
Figure 3. The comparison of PPP, PPG, and PGA between the diabetic and command groups. (A) Peak plantar pressure (PPP); (B) peak pressure gradient (PPG); (C) pressure gradient angle (PGA). T1, first toe; M1, start metatarsal head; M2, second metatarsal head; and HL, heel; *P < 0.05, values are ways with SDs.
The correlations between the PPP, PPG, and PGA are listed in Table i. The correlation between the PPP and PPG was r = 0.58 in the control grouping (P < 0.05) and r = 0.86 in the diabetic group (P < 0.05, Effigy 4A). The correlation between PPG and PGA was r = −0.59 in the control group (P < 0.05) and r = −0.59 in the diabetic group (P < 0.05, Effigy 4B). The correlation between PPP and PGA was significant for the diabetic group (r = −0.44, P < 0.05).
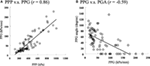
Figure iv. The scatter plots to show the relationships among the PPP, PPG, and PGA in diabetics. (A) The relationship between PPP and PPG, PPP vs. PPG (r = 0.86). (B) The human relationship betwixt PPG and PGA, PPG vs. PGA (r = −0.59).
Discussion
The pathogenesis of diabetic foot ulcers is a multifactorial process that depends on complex interactions between the internal construction and viability of the foot and external forces and pressures during walking (Yarnitzky et al., 2006; Atlas et al., 2009; Lung and Jan, 2012; Jan et al., 2013a). The internal structure of the foot includes geometric shape and alignment of hard and soft tissues, tissue mechanical properties, and private tissue tolerance to loading. The external forces include the dynamic patterns of plantar pressures that can exist characterized by various analyses (e.one thousand., PPP, PPG, and PGA). We believe the PPG may exist a more sensitive indicator of injury take chances than the PPP. The spatial change in pressure across the surface of the skin appears to be an important component of predicting these subsurface shear stresses. We previously defined the top pressure gradient (PPG) as the greatest spatial change in plantar pressure around the PPP location (Fernando et al., 1991).
The location of PPP is identified, and then the spatial change in plantar force per unit area is determined for every direction effectually the PPP location. The greatest spatial change in plantar pressure (i.e., the greatest gradient of the pressure distribution) is the PPG. Based on mechanical theories (Sackfield et al., 2013), we believe pressures that alter substantially across the surface of skin (i.e., loftier PPG) are more damaging than high pressures distributed as across the skin surface. For example, the hydrostatic pressures experienced by the skin of deep-sea divers may exist very high, but divers do not experience skin breakdown because these high pressures are distributed evenly across the surface of the skin (i.due east., they have a very depression PPG). The relationship amidst diverse magnitudes of PPG and the resultant calculated PMSS is illustrated in Effigy 1 using methods previously reported.
In our previous study (Jan et al., 2013a), aberrant PPP and PPG were related to alterations in viscoelastic backdrop of plantar soft tissues of the diabetic foot. Furthermore, diabetics with peripheral neuropathy, who have loss of sensation, may significantly change their gait patterns. Such abnormal gait patterns inevitably alter plantar pressure level distributions in diabetics. The methods and findings of this study aimed to contribute to the understanding of the pathogenesis of diabetic pes ulcers, and our findings alone will non adequate to address the risk of diabetic human foot ulcers.
The results back up our hypotheses that the PPP and PPG of the diabetic group were significantly higher than in the control group at the kickoff toe and outset metatarsal head, and the PGA in the diabetic group were significantly lower than in the command group at the showtime toe. The PGA shows a meaning correlation with the PPG in the diabetic group. The proposed new variable, PGA, was able to further ascertain the force per unit area gradient patterns in diabetics and may provide additional insight into the mechanism of the influences of PPG on the development of diabetic pes ulcers.
Peak plantar force per unit area values were significantly greater in the diabetic group than in the control group at the showtime toe and first metatarsal head. The hateful PPP were greater at the offset toe and first metatarsal head of the diabetic group than in the control group in this study. However, in the heel, no meaning increases were observed in diabetics. These results are consequent with the literature. Perry et al. (2002) showed that the highest plantar pressure level occurred at the first metatarsal head in diabetics. They proposed that the diabetes-associated stiffening of the plantar soft tissues at the pad of the commencement toe and first metatarsal caput may cause this abnormal PPP. The epidermal layer of plantar soft tissues was also reported to go stiffer in diabetics (Chao et al., 2011). Gefen et al. (2001) plant that the stiffness of the soft tissues of the commencement metatarsal head was substantially larger than other plantar regions in diabetics. Zheng et al. (2000b) too demonstrated that the Young'south modulus (elasticity) of plantar tissues of diabetics increased at different plantar areas; the maximum increase (160%) was observed in the area at the start metatarsal head, and the 2d maximum increment was at the first toe, while no significant increase was observed in the heel expanse. Our results back up that the showtime toe and offset metatarsal head are at higher chance for foot ulceration during walking (Gefen, 2003).
The mean PPG were 214% greater in the first toe and 185% in the offset metatarsal caput in the diabetic group than in the control group. The increase in PPG at the first toe and first metatarsal head areas in diabetics may exist attributed to a meaning limitation of move at the metatarsophalangeal joints. The exact pathogenesis of the express joint mobility in diabetics remains unclear but is idea to exist related to the high stiffness of quasi-linear viscoelasticity in the soft tissues (Lung and January, 2012; January et al., 2013a) and the progressive stiffening of the collagen-containing tissues due to accumulation of advanced glycation end products (AGEs) (Burns and Jan, 2012). The diabetic foot with limited motion at the metatarsophalangeal joints significantly reduces shock absorbing ability and may crusade an abnormal plantar pressure distribution (Zimny et al., 2004; D'Ambrogi et al., 2005). Equally illustrated in Figure 2, the PPP alone may not exist able to fully depict the risk of diabetic foot ulcers, and the PPG may provide additional useful data. Fernando et al. (1991) showed that limited joint mobility may be a major factor in causing abnormally high PPP and contributes to foot ulceration. The authors likewise demonstrated that abnormal plantar foot pressures solitary did non predict the location of foot ulcers. Furthermore, Fawzy et al. (2014) indicated that the forefoot PPG in diabetics with neuropathy was ~1.five times higher than that of diabetics without neuropathy. The PPG has previously been reported as related to the posture changes (Hobson, 1992) and materials of insoles modify (Kang and Mak, 1997) in previous studies. The range of movement of the first metatarsophalangeal joint significantly reduced (D'Ambrogi et al., 2005), which may play an of import role of the increased PPG in first toe and first metatarsal caput. Our results of the PPP and PPG in the diabetic and command groups back up the principle of assessing both the PPP and PPG to predict diabetic human foot ulcers.
A noted upshot in this study was that the PPP and PPG of diabetics were non significantly unlike from controls in the heel region and 2d metatarsal head. The reason may be explained by the trajectory of the centre of pressure. Considering shear stress is highly correlated with the PPG (Zou et al., 2007), the trajectory of the center of pressure level passes through the heel region and 2nd metatarsal head during walking (Isle of man et al., 1988). This interpretation is consequent with the results reported by Armstrong et al. (1998b). They reported that merely 1 and 6% of the wounds occurred in the heel region and 2nd metatarsal head. Lord and Hosein (2000) also reported that these 2 regions had lower shear stresses during walking.
The mean PGA was greater at the first toe in the diabetic group than in the control group in this study. Every bit hypothesized, the PGA was significantly lower in diabetics as compared with controls. Ahmed et al. (2010) reported that the almost mutual sites of diabetic foot ulcers were in the plantar surface of the starting time toe. About one-third of diabetics develop a callus at the get-go toe. Plantar callus is associated with high vertical and shear forces in diabetics (Pitei et al., 1999a). When the callus is removed, plantar pressures are reduced by 32.1% in diabetics (Pitei et al., 1999a). This finding indicates that a callus may act equally a strange body elevating plantar pressures. As loftier shear stresses are associated with foot ulcers (Manorama et al., 2010), the low PGA in diabetics may exist negatively related with shear stresses. Further studies need to constitute the relationship between PGA and shear stresses. Calluses are generally non harmful only may sometimes lead to changes in PGA that may aggravate the risk of foot ulceration (Lung and Jan, 2012; January et al., 2013a). To investigate the relationship between calluses and PGA, future research tin can classify the type of influence of PGA on the formation of calluses in response.
Nosotros postulate that the PGA described in this written report tin can be integrated into current risk assessments of diabetic foot ulcers. The PGA has the potential to meliorate the blueprint of orthotic devices in the prevention of diabetic foot ulcers. The correlation between PPG and PPP was considerably higher in the diabetic group than in the control group (r = 0.86 vs. 0.58). As Mueller et al. (2005) defined, the PPG represents the spatial changes in the pressure in the region of the PPP. From a mechanical standpoint, a sharp alter in the highest pressures, i.e., a high PPG, may lead to internal stress concentrations and shearing of soft tissues, causing soft tissue injury (Zhang et al., 1994; Manorama et al., 2010). Although the underlying cause of the increased PPG remains unclear, the involvement of PGA has been implicated. As high PPG appears to have a negative correlation of PGA (r = −0.59), information technology is recommended that further research needs to test and evaluate potential interventions to increase the PGA in diabetics for preventing diabetic foot ulcers. For case, medications may exist used to modify the AGEs accumulation for the reduction of stiffness of plantar soft tissues in diabetics, and thereby possibly reversing the changes in PGA and reducing take a chance for pes ulcers. In addition, orthotic devices may exist designed and constructed to recoup for the changes that cause college PPP and PPG and lower PGA.
This study is a first footstep in comprehensively investigating the importance of the PGA every bit an indicator of diabetic pes ulcers. A benefit of using this approach to estimate PGA is that only the pressure level distribution is needed for information entry. Notwithstanding, PGA requires several assumptions. One of the assumptions is the plantar soft tissue is causeless to be isotropic, homogeneous, and linearly elastic (Jan et al., 2013a). The assumption of small-scale strain deformation is violated because plantar soft tissue deformation can be up to 35–46% (De Clercq et al., 1994; Cavanagh, 1999). PGA was plant to be significantly smaller in the diabetic group in T1. The soft tissue depth may affect the values of PGA. Zheng et al. (2000a) showed that the tissue thickness of the T1 is thinner than M1, M2, and HL. PGA may thus play a significant role in thinner soft tissue of diabetes foot.
At that place are several limitations of this study. Offset, the sample size was small, which might impede the power of the statistical analysis. Nosotros did not have an age and BMI matched control. Factors, such equally historic period and BMI, may affect our results. Nosotros performed a correlation analysis to examine whether demographic data (age, trunk weight, and BMI) significantly contributed to the results observed in this study. We did not find whatever pregnant correlation between the demographic data and variables of this written report. Although the diabetic group was 27 kg heavier than the not-diabetic grouping, obesity and beingness overweight are unremarkably observed in the type 2 diabetics and may inherently contribute to abnormal plantar pressure distributions (Qatanani and Lazar, 2007; Atlas et al., 2009). Cavanagh et al. (1991) demonstrated that there was lack of a strong relationship between body weight and plantar pressure parameters. We did not perform a longitudinal follow-up of the incidence of diabetic pes ulcer to examine the power of using the PGA on predicting foot ulcers. Whether changes in PGA are associated with a higher risk for pes ulcers require additional investigation.
Conclusion
Nosotros introduced PGA to further quantify the force per unit area gradient patterns in diabetics in this study and successfully demonstrated that diabetics take higher PPP and PPG, and lower PGA particularly at the first toe compared with non-diabetics. Our method and findings may contribute to the understanding of the role of plantar pressures in the development of diabetic human foot ulcers. Our findings provide a basis to further explore the fourth dimension-varying features of plantar pressure distributions associated with the functional and structural changes of the diabetic foot.
Author Contributions
Study concept and blueprint: Y-KJ. Conquering of information: C-WL. Analysis and interpretation of information: C-WL, EH-W, SB, FL, and Y-KJ. Drafting of the manuscript: C-WL and Y-KJ. Disquisitional revision of manuscript for important intellectual content: C-WL, EH-W, SB, FL, and Y-KJ. Obtained funding: Y-KJ.
Conflict of Interest Statement
The authors declare that the inquiry was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by the Oklahoma Center for the Advancement of Science and Technology (HR09-048).
References
Ahmed, One thousand. E., Tamimi, A. O., Mahadi, S. I., Widatalla, A. H., and Shawer, M. A. (2010). Hallux ulceration in diabetic patients. J. Foot Ankle Surg. 49, two–vii. doi: 10.1053/j.jfas.2009.07.005
PubMed Abstract | CrossRef Full Text | Google Scholar
Apelqvist, J., Bakker, G., van Houtum, West. H., Nabuurs-Franssen, M. H., and Schaper, N. C. (2000). International consensus and practical guidelines on the management and the prevention of the diabetic foot. International Working Grouping on the Diabetic Foot. Diabetes Metab. Res. Rev. 16(Suppl. 1), S84–S92. doi:10.1002/1520-7560(200009/10)16:1+<::AID-DMRR113>3.0.CO;2-Due south
CrossRef Full Text | Google Scholar
Armstrong, D. G., Lavery, L. A., and Bushman, T. R. (1998a). Summit foot pressures influence the healing fourth dimension of diabetic foot ulcers treated with total contact casts. J. Rehabil. Res. Dev. 35, one–v.
PubMed Abstract | Google Scholar
Armstrong, D. K., Lavery, L. A., and Harkless, L. B. (1998b). Validation of a diabetic wound nomenclature organisation: the contribution of depth, infection, and ischemia to run a risk of amputation. Diabetes Care 21, 855–859. doi:10.2337/diacare.21.5.855
PubMed Abstract | CrossRef Full Text | Google Scholar
Atlas, E., Yizhar, Z., Khamis, S., Slomka, N., Hayek, S., and Gefen, A. (2009). Utilization of the pes load monitor for evaluating deep plantar tissue stresses in patients with diabetes: proof-of-concept studies. Gait Posture 29, 377–382. doi:10.1016/j.gaitpost.2008.x.055
PubMed Abstract | CrossRef Total Text | Google Scholar
Burns, Southward., and Jan, Y. K. (2012). "Diabetic foot ulceration and amputation," in Rehabilitation Medicine, 1st Edn, ed. C. T. Kim (Republic of croatia: InTech Publisher), 1–20.
Google Scholar
Caselli, A., Pham, H., Giurini, J. G., Armstrong, D. G., and Veves, A. (2002). The forefoot-to-rearfoot plantar pressure ratio is increased in severe diabetic neuropathy and tin predict pes ulceration. Diabetes Care 25, 1066–1071. doi:10.2337/diacare.25.six.1066
PubMed Abstract | CrossRef Total Text | Google Scholar
Cavanagh, P. R., Sims, D. Southward., and Sanders, 50. J. (1991). Trunk mass is a poor predictor of tiptop plantar force per unit area in diabetic men. Diabetes Intendance xiv, 750–755. doi:ten.2337/diacare.14.8.750
PubMed Abstract | CrossRef Full Text | Google Scholar
Chao, C. Y. 50., Zheng, Y. P., and Cheing, Thousand. L. Y. (2011). Epidermal thickness and biomechanical properties of plantar tissues in diabetic pes. Ultrasound Med. Biol. 37, 1029–1038. doi:10.1016/j.ultrasmedbio.2011.04.004
PubMed Abstract | CrossRef Total Text | Google Scholar
Chen, W. K., Lee, T., Lee, P. V. Due south., Lee, J. W., and Lee, S. J. (2010). Effects of internal stress concentrations in plantar soft-tissue – a preliminary three-dimensional finite chemical element analysis. Med. Eng. Phys. 32, 324–331. doi:10.1016/j.medengphy.2010.01.001
CrossRef Full Text | Google Scholar
D'Ambrogi, E., Giacomozzi, C., Macellari, Five., and Uccioli, L. (2005). Abnormal foot function in diabetic patients: the altered onset of windlass machinery. Diabet. Med. 22, 1713–1719. doi:x.1111/j.1464-5491.2005.01699.10
PubMed Abstract | CrossRef Total Text | Google Scholar
De Clercq, D., Aerts, P., and Kunnen, M. (1994). The mechanical characteristics of the human heel pad during foot strike in running: an in vivo cineradiographic study. J. Biomech. 27, 1213–1222. doi:10.1016/0021-9290(94)90275-5
PubMed Abstract | CrossRef Full Text | Google Scholar
Fawzy, O. A., Arafa, A. I., El Wakeel, M. A., and Abdul Kareem, South. H. (2014). Plantar pressure as a adventure assessment tool for diabetic human foot ulceration in Egyptian patients with diabetes. Clin. Med. Insights Endocrinol. Diabetes vii, 31–39. doi:ten.4137/cmed.s17088
PubMed Abstract | CrossRef Full Text | Google Scholar
Fernando, D. J., Masson, E. A., Veves, A., and Boulton, A. J. (1991). Human relationship of limited joint mobility to abnormal foot pressures and diabetic human foot ulceration. Diabetes Care 14, viii–11. doi:10.2337/diacare.14.1.eight
PubMed Abstract | CrossRef Full Text | Google Scholar
Gefen, A., Megido-Ravid, Grand., Azariah, M., Itzchak, Y., and Arcan, K. (2001). Integration of plantar soft tissue stiffness measurements in routine MRI of the diabetic foot. Clin. Biomech. 16, 921–925. doi:x.1016/s0268-0033(01)00074-2
PubMed Abstract | CrossRef Total Text | Google Scholar
Gefen, A., Megido-Ravid, Grand., Itzchak, Y., and Arcan, M. (2000). Biomechanical analysis of the iii-dimensional foot structure during gait: a basic tool for clinical applications. J. Biomech. Eng. 122, 630–639. doi:x.1115/one.1318904
PubMed Abstract | CrossRef Full Text | Google Scholar
Jan, Y. K., Lung, C. W., Cuaderes, E., Rong, D., and Boyce, Grand. (2013a). Effect of viscoelastic backdrop of plantar soft tissues on plantar pressures at the first metatarsal head in diabetics with peripheral neuropathy. Physiol. Meas. 34, 53–66. doi:x.1088/0967-3334/34/1/53
PubMed Abstract | CrossRef Total Text | Google Scholar
Jan, Y. K., Shen, S., Foreman, R. D., and Ennis, West. J. (2013b). Pare blood period response to locally practical mechanical and thermal stresses in the diabetic human foot. Microvasc. Res. 89, forty–46. doi:x.1016/j.mvr.2013.05.004
PubMed Abstract | CrossRef Total Text | Google Scholar
Kang, T. E., and Mak, A. F. (1997). Evaluation of a simple arroyo to modify the supporting property of seating foam cushion for pressure level relief. Help. Technol. nine, 47–54. doi:ten.1080/10400435.1997.10132295
PubMed Abstract | CrossRef Full Text | Google Scholar
Klaesner, J. West., Hastings, M. Chiliad., Zou, D., Lewis, C., and Mueller, M. J. (2002). Plantar tissue stiffness in patients with diabetes mellitus and peripheral neuropathy. Arch. Phys. Med. Rehabil. 83, 1796–1801. doi:ten.1053/apmr.2002.35661
PubMed Abstruse | CrossRef Full Text | Google Scholar
Lavery, L. A., Armstrong, D. G., Wunderlich, R. P., Tredwell, J., and Boulton, A. J. Yard. (2003). Predictive value of foot pressure assessment every bit part of a population-based diabetes illness direction programme. Diabetes Intendance 26, 1069–1073. doi:10.2337/diacare.26.four.1069
PubMed Abstruse | CrossRef Full Text | Google Scholar
Lord, M., and Hosein, R. (2000). A written report of in-shoe plantar shear in patients with diabetic neuropathy. Clin. Biomech. (Bristol, Avon) xv, 278–283. doi:10.1016/S0268-0033(99)00076-5
CrossRef Total Text | Google Scholar
Lott, D. J., Zou, D., and Mueller, 1000. J. (2008). Pressure slope and subsurface shear stress on the neuropathic forefoot. Clin. Biomech. (Bristol, Avon) 23, 342–348. doi:10.1016/j.clinbiomech.2007.10.005
PubMed Abstract | CrossRef Full Text | Google Scholar
Lung, C. W., and Jan, Y. K. (2012). "Soft tissue biomechanics of diabetic foot ulcers," in Soft Tissue: Composition, Mechanisms of Injury and Repair, 1st Edn, eds A. J. C. Ruiz and J. M. A. Mendoza (Hauppauge, NY: Nova Science Publishers), 1–32.
Google Scholar
Lung, C. W., Liau, B. Y., and Jan, Y. Grand. (2013). Plantar pressure gradient angles to evaluate risk of diabetic foot ulcer. Lect. Notes Comput. Sci. 8025, 240–247. doi:10.1007/978-3-642-39173-6_29
CrossRef Total Text | Google Scholar
Mak, A. F., Zhang, M., and Tam, E. Westward. (2010). Biomechanics of pressure ulcer in body tissues interacting with external forces during locomotion. Annu. Rev. Biomed. Eng. 12, 29–53. doi:10.1146/annurev-bioeng-070909-105223
PubMed Abstruse | CrossRef Full Text | Google Scholar
Isle of mann, R. A., Poppen, North. K., and O'Konski, M. (1988). Amputation of the not bad toe. A clinical and biomechanical study. Clin. Orthop. Relat. Res. 226, 192–205.
Google Scholar
Manorama, A. A., Baek, S., Vorro, J., Sikorskii, A., and Bush-league, T. R. (2010). Blood perfusion and transcutaneous oxygen level characterizations in human skin with changes in normal and shear loads – implications for pressure ulcer formation. Clin. Biomech. 25, 823–828. doi:ten.1016/j.clinbiomech.2010.06.003
CrossRef Total Text | Google Scholar
Mueller, Thousand. J., Zou, D., Bohnert, Thou. L., Tuttle, Fifty. J., and Sinacore, D. R. (2008). Plantar stresses on the neuropathic human foot during barefoot walking. Phys. Ther. 88, 1375. doi:10.2522/ptj.20080011
PubMed Abstruse | CrossRef Total Text | Google Scholar
Mueller, M. J., Zou, D., and Lott, D. J. (2005). "Pressure slope" as an indicator of plantar skin injury. Diabetes Care 28, 2908–2912. doi:10.2337/diacare.28.12.2908
CrossRef Full Text | Google Scholar
Patry, J., Belley, R., Cote, Thousand., and Chateau-Degat, M. L. (2013). Plantar pressures, plantar forces, and their influence on the pathogenesis of diabetic pes ulcers: a review. J. Am. Podiatr. Med. Assoc. 103, 322–332. doi:10.7547/1030322
PubMed Abstract | CrossRef Total Text | Google Scholar
Perry, J. E., Hall, J. O., and Davis, B. L. (2002). Simultaneous measurement of plantar pressure and shear forces in diabetic individuals. Gait Posture 15, 101–107. doi:x.1016/s0966-6362(01)00176-10
PubMed Abstract | CrossRef Full Text | Google Scholar
Pitei, D. Fifty., Lord, Thousand., Foster, A., Wilson, S., Watkins, P. J., and Edmonds, M. East. (1999b). Plantar pressures are elevated in the neuroischemic and the neuropathic diabetic foot. Diabetes Care 22, 1966–1970. doi:10.2337/diacare.22.12.1966
PubMed Abstruse | CrossRef Full Text | Google Scholar
Sackfield, A., Hills, D., and Nowell, D. (2013). Mechanics of Elastic Contacts. Amsterdam: Elsevier.
Google Scholar
Stockl, K., Vanderplas, A., Tafesse, E., and Chang, E. (2004). Costs of lower-extremity ulcers among patients with diabetes. Diabetes Care 27, 2129–2134. doi:x.2337/diacare.27.ix.2129
PubMed Abstract | CrossRef Full Text | Google Scholar
Supriadi, Chiliad., Nishizawa, T., Fukuda, M., Kon, Y., Junko, M., Suriadi, Thou., et al. (2014). Interface pressure, force per unit area gradient with pressure ulcer development in intensive intendance units. J. Nurs. Educ. Prac. 4, 146. doi:10.5430/jnep.v4n9p146
CrossRef Full Text | Google Scholar
Veves, A., Murray, H. J., Young, M. J., and Boulton, A. J. (1992). The risk of foot ulceration in diabetic patients with high foot pressure: a prospective study. Diabetologia 35, 660–663. doi:10.1007/BF00400259
PubMed Abstruse | CrossRef Total Text | Google Scholar
Yarnitzky, Grand., Yizhar, Z., and Gefen, A. (2006). Real-time subject-specific monitoring of internal deformations and stresses in the soft tissues of the foot: a new approach in gait analysis. J. Biomech. 39, 2673–2689. doi:10.1016/j.jbiomech.2005.08.021
PubMed Abstract | CrossRef Total Text | Google Scholar
Zhang, M., Turner-Smith, A. R., and Roberts, V. C. (1994). The reaction of skin and soft tissue to shear forces applied externally to the pare surface. Proc. Inst. Mech. Eng. H 208, 217–222. doi:10.1243/pime_proc_1994_208_291_02
CrossRef Full Text | Google Scholar
Zheng, Y. P., Choi, Y. Chiliad., Wong, G., Chan, S., and Mak, A. F. (2000a). Biomechanical cess of plantar foot tissue in diabetic patients using an ultrasound indentation system. Ultrasound Med. Biol. 26, 451–456. doi:10.1016/S0301-5629(99)00163-5
PubMed Abstract | CrossRef Full Text | Google Scholar
Zheng, Y. P., Choi, Y. K., Wong, K., Chan, S., and Mak, A. F. T. (2000b). Biomechanical assessment of plantar human foot tissue in diabetic patients using an ultrasound indentation system. Ultrasound Med. Biol. 26, 451–456. doi:10.1016/S0301-5629(99)00163-5
PubMed Abstruse | CrossRef Full Text | Google Scholar
Zou, D., Mueller, M. J., and Lott, D. J. (2007). Consequence of peak force per unit area and pressure level slope on subsurface shear stresses in the neuropathic human foot. J. Biomech. 40, 883–890. doi:10.1016/j.jbiomech.2006.03.005
PubMed Abstract | CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fbioe.2016.00054/full
0 Response to "A Review of the Biomechanics of the Diabetic Foot"
Post a Comment